Understanding FDA Authorization, Safety Claims, and What Consumers Should Know
As disposable vapes continue to grow in popularity, many consumers search for answers to one critical question: What disposable vapes are FDA-approved? Closely related questions include What vapes are approved by the FDA? and What is the safest disposable vape on the market?
These questions are understandable—but they are also based on a common misunderstanding. The U.S. Food and Drug Administration (FDA) does not approve electronic cigarettes in the same way it approves medications or medical devices. Instead, vaping products fall under a regulatory authorization system, not a safety certification system.
This article explains what FDA authorization actually means, whether any disposable vapes are authorized, how to identify misleading “FDA approved” claims, and how consumers should think about safety in realistic terms.

Key Takeaways
- The FDA does not approve disposable vapes like medicines
- Most disposable vapes are not FDA-authorized
- Authorization does not equal safety
- “FDA-approved vape” is usually a misleading phrase
- There is no officially recognized “safest disposable vape.”
- Understanding regulation helps consumers avoid false claims
Why People Search for “FDA Approved Disposable Vapes”
Disposable vapes are often marketed as convenient, modern, and cleaner alternatives to cigarettes. At the same time, users are increasingly concerned about safety, legality, and product quality.
Search intent behind this topic usually includes:
- Avoiding illegal or unsafe products
- Finding products perceived as “officially approved.”
- Reducing health risks compared to smoking
However, the phrase “FDA-approved vape” is misleading and, in many cases, inaccurate.
Does the FDA Actually “Approve” Disposable Vapes?
No. The FDA does not “approve” vapes the way it approves drugs, vaccines, or medical devices.
Instead, the FDA regulates vaping products as tobacco products under the Family Smoking Prevention and Tobacco Control Act. For a vape product to be legally marketed in the U.S., manufacturers must submit a Premarket Tobacco Product Application (PMTA).
If the FDA determines that allowing the product to be sold is appropriate for the protection of public health, it may grant marketing authorization.
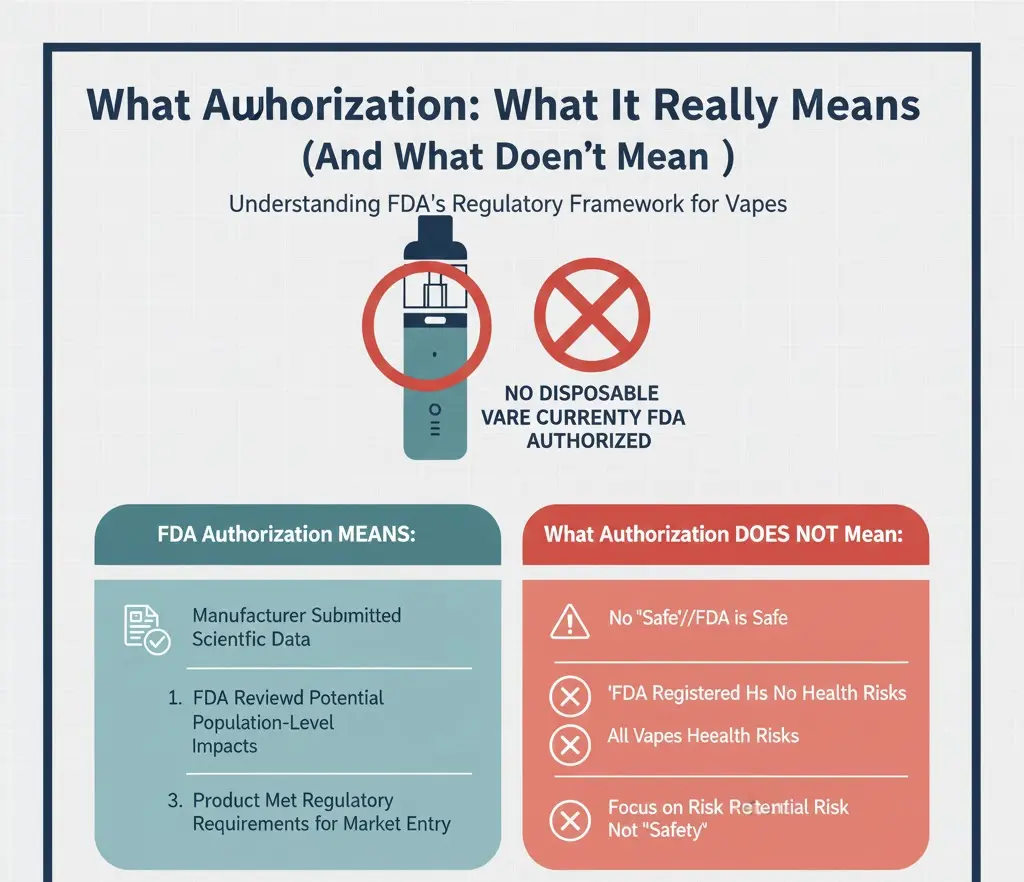
Important distinction: Authorization does not mean the product is safe, harmless, or recommended.
What Does FDA Authorization Actually Mean?
FDA authorization means:
- The manufacturer submitted scientific data
- The FDA reviewed potential population-level impacts
- The product met regulatory requirements for market entry
FDA authorization does not mean:
- The product is safe to use
- The product has no health risks
- The product is recommended for non-smokers
The FDA consistently states that no tobacco product is safe, including electronic cigarettes.
FDA Terminology Explained (Important Table)
This table helps clarify the most misunderstood FDA-related terms used in vape marketing.
| Term | What It Means | Common Misuse |
| FDA Approved | Used for drugs & medical devices | Incorrectly applied to vapes |
| FDA Authorized | PMTA granted for market entry | Often simplified as “approved” |
| FDA Registered | Manufacturer facility registered | Does NOT mean product reviewed |
| FDA Compliant | Vague marketing term | No official FDA definition |
Key point: If a vape claims to be “FDA approved,” that claim is almost always misleading.
What Vapes Are Authorized by the FDA?
Authorized Vape Products (General Overview)
As of now, the FDA has authorized a limited number of electronic nicotine delivery systems, primarily:
- Tobacco-flavored products
- Closed-system or cartridge-based devices
- Products with extensive scientific documentation
These authorizations are rare, highly specific, and subject to ongoing review.
Are Any Disposable Vapes FDA Authorized?
In practical terms:
- Very few (if any) typical flavored disposable vapes have received FDA marketing authorization
- Most disposable vapes currently on the market are either:
- Pending review
- Denied authorization
- Being sold under the enforcement discretion
Disposable devices face additional regulatory challenges due to:
- High youth appeal
- Flavored formulations
- Lack of refill control
- Difficulty limiting misuse
Authorization status can also change over time, which is why responsible articles avoid listing brand names as “FDA approved.”
Why Most Disposable Vapes Are Not FDA-Approved
Several factors make FDA authorization difficult for disposable products:
- Flavor Restrictions
Flavored vapes are closely scrutinized due to youth usage concerns. - Population Health Standard
FDA evaluates impact on smokers and non-smokers—not just individual users. - High Data Requirements
PMTA submissions require toxicology, chemistry, and behavioral studies. - Enforcement Priorities
The FDA focuses its enforcement on unauthorized products, especially those appealing to minors.
What Is the Safest Disposable Vape on the Market?
This is one of the most searched questions—but it has no official answer.
There is no FDA-designated “safest disposable vape.” Safety is relative and depends on context.
From a harm-reduction perspective:
- For adult smokers who fully switch, vaping may reduce exposure to certain harmful combustion byproducts
- For non-smokers, disposable vapes introduce avoidable health risks
Factors that influence relative risk include:
- Nicotine strength
- Frequency of use
- User age and health status
- Complete switching vs dual use
“Safer than smoking” does not mean “safe.”

How to Identify Misleading “FDA Approved Vape” Claims (Detailed Guide)
Many consumers search for “FDA-approved disposable vapes” because they want a product that feels official, legal, and safer. Unfortunately, “FDA-approved vape” is a common marketing phrase—and often a misleading one.
The FDA does not “approve” vapes like medications. For vapes, the key concept is marketing authorization (a written marketing order), usually through the PMTA pathway. To protect yourself from misleading claims, use the checklist below.
1) Know the 3 Most Common Misleading Phrases (And What They Usually Mean)
Misleading Phrase A: “FDA Approved”
- Why it’s a red flag: “FDA approved” is mainly a medical-product standard. For vapes, the correct concept is a marketing order/authorization.
- What it often really means: The seller is trying to signal “legal” or “safe” without proving authorization.
Tip: If the product page only says “FDA approved” with no order letter, no product listing, and no official reference, treat it as unreliable.
Misleading Phrase B: “FDA Certified” / “FDA Verified”
- Why it’s a red flag: These are not standard FDA categories for vapes.
- What it often really means: A vague credibility cue with no regulatory substance.
Misleading Phrase C: “FDA Guaranteed Safe” / “Doctor Recommended” / “Safe for Your Lungs”
- Why it’s a red flag: The FDA has taken action against vaping products marketed with unproven health/wellness claims.
- What it often really means: A health claim that may be illegal or unsupported.
Tip: If a vape is marketed as a health aid, wellness device, or “safe,” that’s a major EEAT warning sign.
2) The “Good” Language You Want to See Instead
A more credible product or brand will use language like:
- “FDA authorized” or “FDA marketing authorization.”
- “Marketing Granted Order (MGO)”
- “Authorized through the PMTA pathway” …and ideally provide proof (see the next section).
FDA explains that a product with an MGO under PMTA can be legally marketed (subject to the order’s conditions).
3) How to Verify Claims (Step-by-Step)
You don’t need to “trust the brand.” You can verify with official FDA resources.
Step 1 — Check the FDA’s “E-cigarettes Authorized by the FDA” List
FDA maintains an up-to-date list of e-cigarettes authorized by the FDA.
What to look for:
- Manufacturer name
- Product name (exact match)
- Device type (closed system, pods, etc.)
Red flag: If the brand claims FDA authorization but is not listed (or cannot point you to an official FDA list entry), the claim is questionable.
Step 2 — Look for a Written Marketing Order / Marketing Granted Order
The FDA explains that to legally market a new tobacco product, a company must receive a written marketing order via PMTA/SE/EX REQ pathways.
Tip: Credible brands often reference “marketing order,” “order letter,” or “MGO,” not “approved.”
Step 3 — Use the Searchable Tobacco Products Database
FDA provides additional information about its Searchable Tobacco Products Database, including what an MGO means. This can help confirm whether a product has an authorization record.
Red flag: “We are FDA registered” is not the same as “our product is authorized.”
4) Don’t Get Tricked by “FDA Registered” (This Is a Big One)
Some sellers say:
- “FDA registered facility.”
- “FDA-registered products.”
- “Manufactured in an FDA-registered lab.”
Why it’s misleading: “Registered” typically refers to a facility or administrative listing—not proof that the specific vape product has an FDA marketing order.
Rule of thumb: If the claim does not clearly connect to a specific authorized product listing or marketing order, it doesn’t prove legality or safety.
5) Spot “Legality Theater” on Product Pages (Common Red Flags)
Here are patterns that frequently appear in misleading listings:
- No specific product name matches the FDA list
- No manufacturer details
- “FDA approved” repeated, but no links to FDA pages
- Overuse of safety language:
- “100% safe.”
- “harmless vapor.”
- “safe for lungs.”
- Claims that sound like medical benefits:
- “helps anxiety”
- “improves focus”
- “treats asthma”
The FDA has explicitly warned consumers about vaping products being sold with unproven health claims.
6) Why This Matters: Unauthorized Products Are a Real Enforcement Target
The FDA regularly issues warning letters and enforcement actions against unauthorized e-cigarette products and retailers selling them. That’s important because it shows “everyone sells it” does not equal “it’s authorized.”
Tip: If a product is marketed heavily as a flavored disposable and claims “FDA approved,” be extra cautious—those categories are frequently involved in enforcement actions.
Quick Consumer Checklist (Copy/Paste Box)
Before trusting an “FDA-approved vape” claim, ask:
- Does the listing say FDA authorized (not just “approved”)?
- Can the seller show the product on the FDA-authorized e-cigarette list?
- Is there mention of a marketing order/MGO?
- Are there health or safety guarantees that sound too absolute?
- Is the language specific, transparent, and verifiable—or vague and promotional?
If most answers are “no,” treat the claim as unreliable.
FDA Regulation vs Consumer Safety
FDA regulation focuses on market authorization, not personal health endorsement.
A product can be:
- Legal to sell
- Fully authorized
- Still harmful to use
This is why the FDA repeatedly emphasizes that non-users should not start using tobacco products, including vapes.
FAQ – FDA-Approved Disposable Vapes
Are any disposable vapes FDA-approved?
No. The FDA does not approve vapes. Some products may receive marketing authorization, but this is not an approval or safety certification.
What vapes are authorized by the FDA?
Only a limited number of electronic nicotine products—primarily tobacco-flavored and closed systems—have received authorization.
Is there the safest disposable vape?
There is no officially designated safest disposable vape. All carry health risks.
Does FDA authorization mean a vape is safe?
No. Authorization only means the product met regulatory requirements for market entry.
Why do some vapes claim to be FDA-approved?
This is often marketing language or a misunderstanding. FDA approval does not apply to vaping products.
See more
How to Fix a Disposable Vape That Won’t Hit
What Is a Dry Herb Vape? How They Work, and How They Compare to Smoking
How to Use a Disposable Vape Pen with a Button: A Beginner’s Step-by-Step Guide
